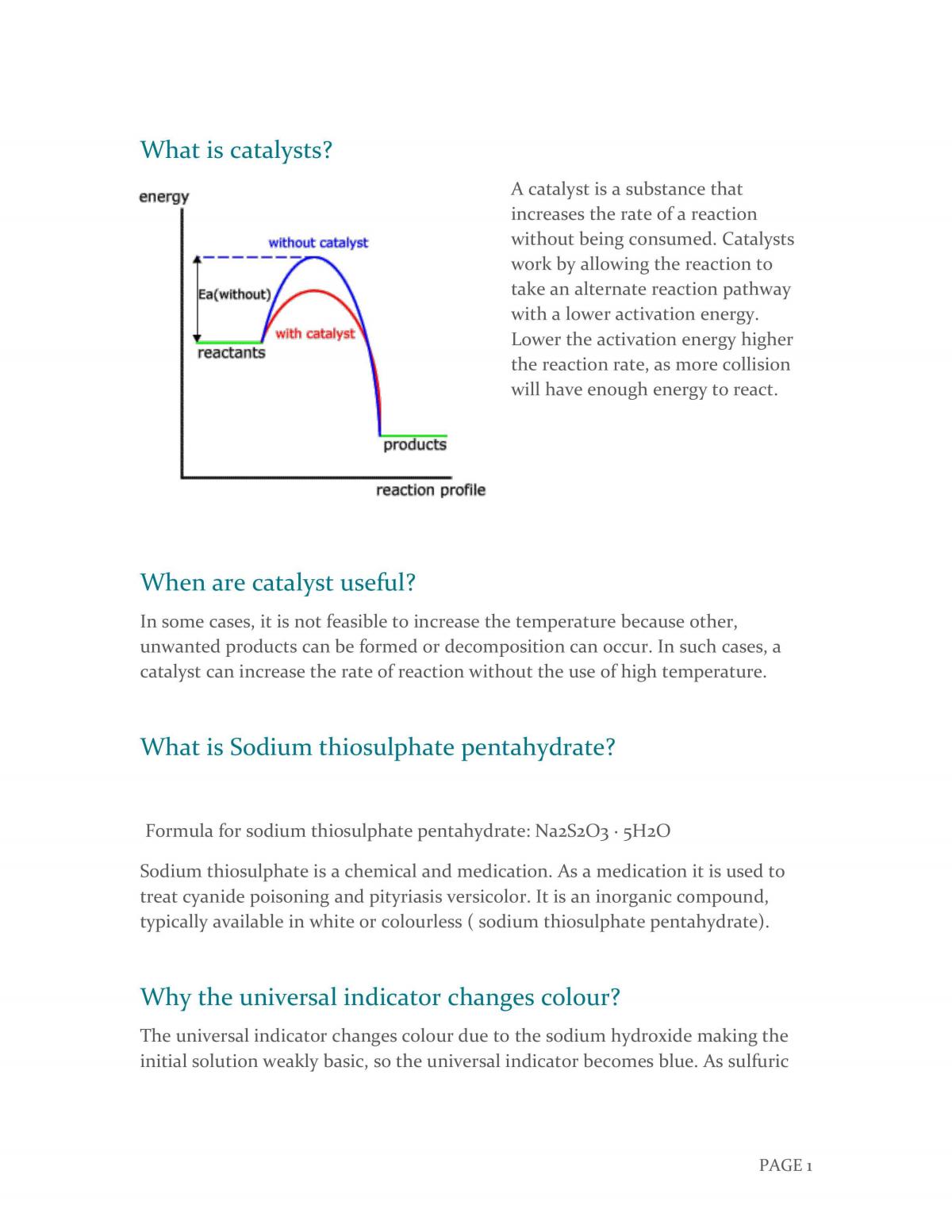
Catalysis of the Reaction Between Sodium Thiosulphate Pentahydrate and Hydrogen Peroxide | Chemistry - Year 11 HSC | Thinkswap

Oxygen−Sulfur Species Distribution and Kinetic Analysis in the Hydrogen Peroxide−Thiosulfate System | Inorganic Chemistry
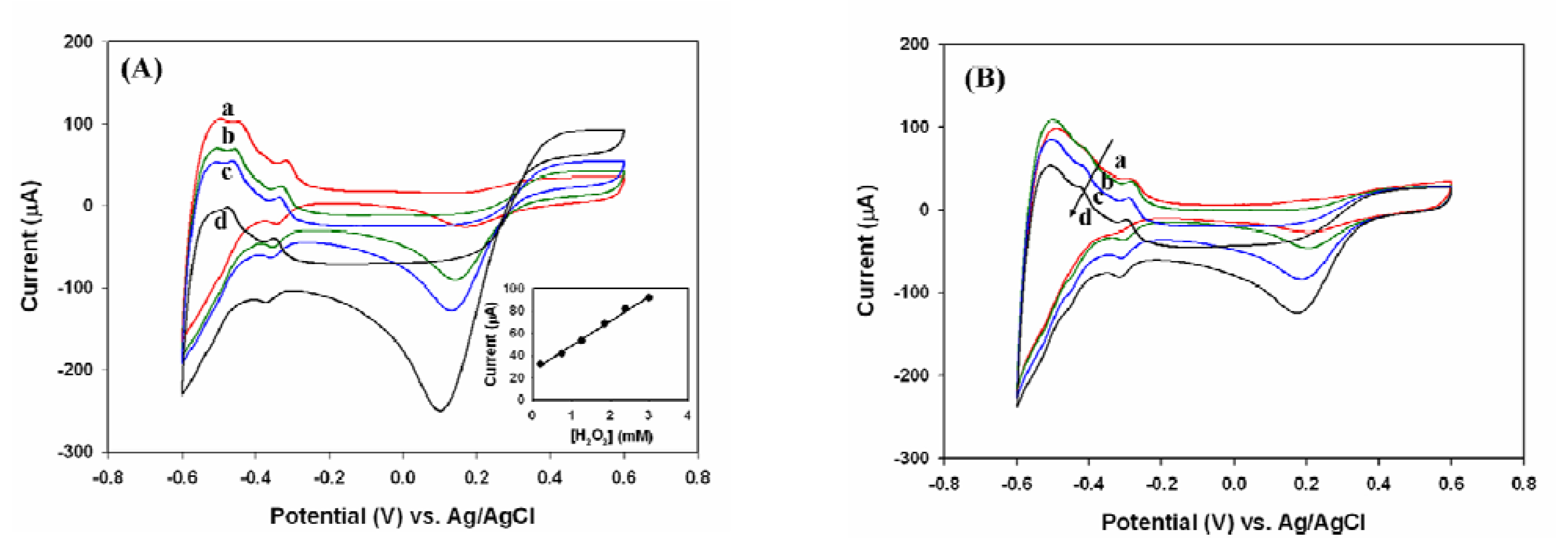
Sensors | Free Full-Text | Eliminating the Interference of Oxygen for Sensing Hydrogen Peroxide with the Polyaniline Modified Electrode

Oxygen−Sulfur Species Distribution and Kinetic Analysis in the Hydrogen Peroxide−Thiosulfate System | Inorganic Chemistry
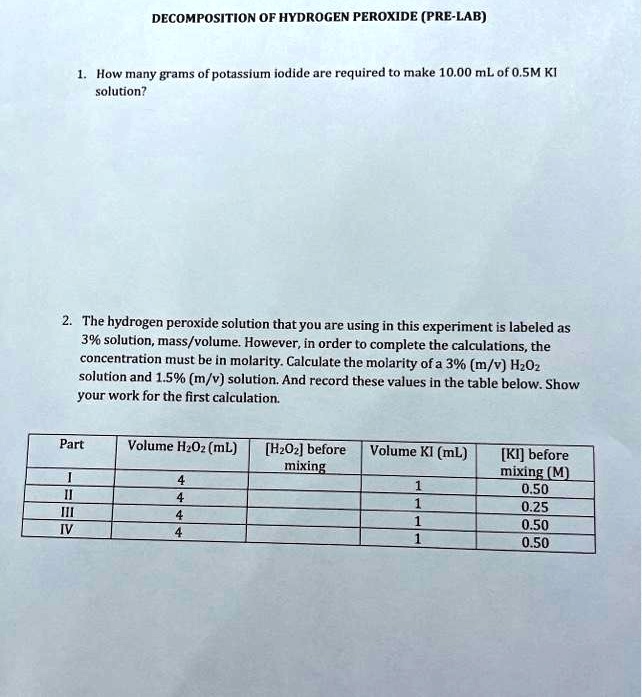
SOLVED: DECOMPOSITION OF HYDROGEN PEROXIDE (PRE-LAB) How many grams of potassium iodide are required to make 10.00 mLofO5M KI solution? The hydrogen peroxide solution thatyou are using in this experiment is labeled

A.) Chemical reaction between hydrogen peroxide and potassium iodide.... | Download Scientific Diagram
The use of sodium thiosulfate for inactivating residual hydrogen peroxide on contact lenses after disinfection

Rate of Decomposition of Hydrogen Peroxide (1.4.1) | OCR A Level Chemistry Revision Notes 2017 | Save My Exams

Oxygen−Sulfur Species Distribution and Kinetic Analysis in the Hydrogen Peroxide−Thiosulfate System | Inorganic Chemistry

Mechanism of the oxidation of thiosulfate with hydrogen peroxide catalyzed by aqua-ethylenediaminetetraacetatoruthenium(III) - ScienceDirect
Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen Peroxide Molecular equation: 2KI(aq) + 2HCl(aq) + H O (aq) I (s) +
Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen Peroxide Molecular equation: 2KI(aq) + 2HCl(aq) + H O (aq) I (s) +